The DEEP project has received research funding from the European Union under the 7th Framework Programme
DEEP is a 6-year European Project (FP7) coordinated by Consorzio per Valutazioni Biologiche e Farmacologiche (CVBF), comprising 23 recruiting centres in European and non-European countries, scientific partners from several European countries and a pharmaceutical group based in Canada. The DEEP Project has been developed with the specific intent to integrate the existing information on deferiprone use in paediatric patients, thus covering the lack of information and providing a valid support to the use of the drug in this class of age. The aim of DEEP is to provide data on deferiprone pharmacokinetics in younger children, to evaluate the safety of deferiprone in the clinical setting through a long-term observational study and to generate new comparative efficacy/safety data to be used to grant a Marketing Authorisation (MA) of a new liquid formulation of the drug, specifically developed for children use.
Latest News
DEEP-1
Performing a pharmacokinetics trial of deferiprone in children up to 6 years
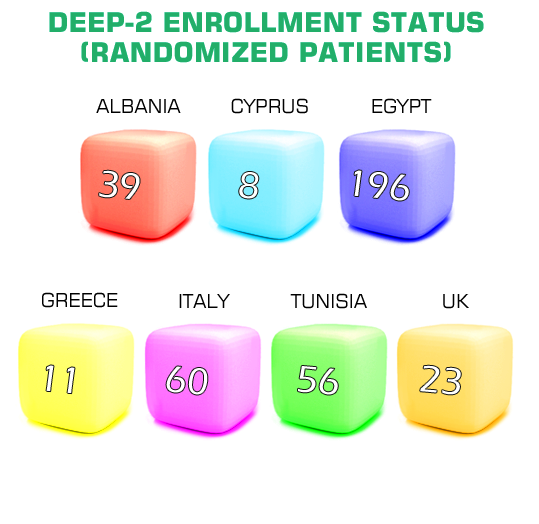
DEEP-2
Performing an efficacy/safety trialto compare deferiprone with deferasirox
DEEP-3
Performing a long-term safety study of deferiprone use in paediatric patients