The most important achievements and activities carried out by the project have been collected and included in the DEEP Scientific Report related to the period from 01/01/2014 to 30/06/2015 which has been submitted to the European Commission on September 2015.
The report provided a comprehensive overview of the activities performed with particular reference to the implementation of the DEEP studies in compliance with the Paediatric Investigation Plan (EMEA-001126-PIP01-10), as approved and further amended by the Paediatric Committee (PDCO).
The main efforts of this reporting period have been devoted to implement DEEP 2 recruitment and study conduct. In particular, in order to reach the required patient number, the number of clinical centers has been increased, recruitment capacity has been checked and study qualification visits have been performed.
As stated in the report, even if the main project activities have been completed and the great majority of the deliverables and milestones foreseen for that period have been addressed, many delays have occurred mainly due to the lengthy approval process imposed by many local ethics committees and competent authorities. These problems have led to a request of amendment of the DEEP Grant Agreement, which has been approved on 10.06.2015 by the European Commission providing an extension of 20 months of the project. The specific reasons for requesting this extension have been:
• prolonged difficulties due to the political instability in two of the North African countries, Egypt and Tunisia, which were representing over 50% of the total potential patients recruitment;
• need to identify new recruiting centres;
• changes in Regulatory Procedures for authorisation of the studies in different countries;
• changes in the DEEP 2 study protocol as included in a modified agreed PIP.
Furthermore, DEEP has also faced many regulatory issues that led on 26/08/2014 (following a Letter of intent on June 2014) to a request for modification (RfM) of the agreed PIP to the European Medicines Agency (EMA) and its Paediatric Committee (PDCO). The procedure ended on 17/11/2014 as described in the figure below.
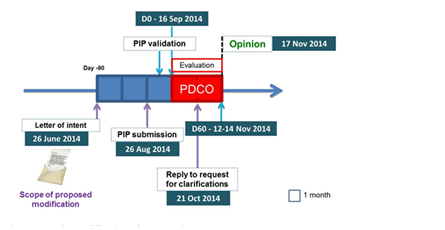 The PIP modification was motivated by the need to implement the results of the modification introduced by the scientific coordination group into the DEEP 2 protocol as well as it was intended to clarify minimal aspects on timing and use of data with reference to DEEP-1 and DEEP-3 studies. In this context and to create the conditions for operating according to the European ethical and regulatory legislation, the DEEP Coordinator has developed specific standard operating procedures (SOPs) and guidelines to be implemented by all DEEP Beneficiaries.
The PIP modification was motivated by the need to implement the results of the modification introduced by the scientific coordination group into the DEEP 2 protocol as well as it was intended to clarify minimal aspects on timing and use of data with reference to DEEP-1 and DEEP-3 studies. In this context and to create the conditions for operating according to the European ethical and regulatory legislation, the DEEP Coordinator has developed specific standard operating procedures (SOPs) and guidelines to be implemented by all DEEP Beneficiaries.
As a result, the DEEP project’s commitment of operating according to the European ethical and regulatory legislation has ensured and will still guarantee the implementation of ethics principles and GCP recommendations in all participating centres, including those located outside the European area, thus promoting the spread of such practices in clinical research.

