![]() Albania – Tirana is ready to enrol its first patient
Albania – Tirana is ready to enrol its first patient
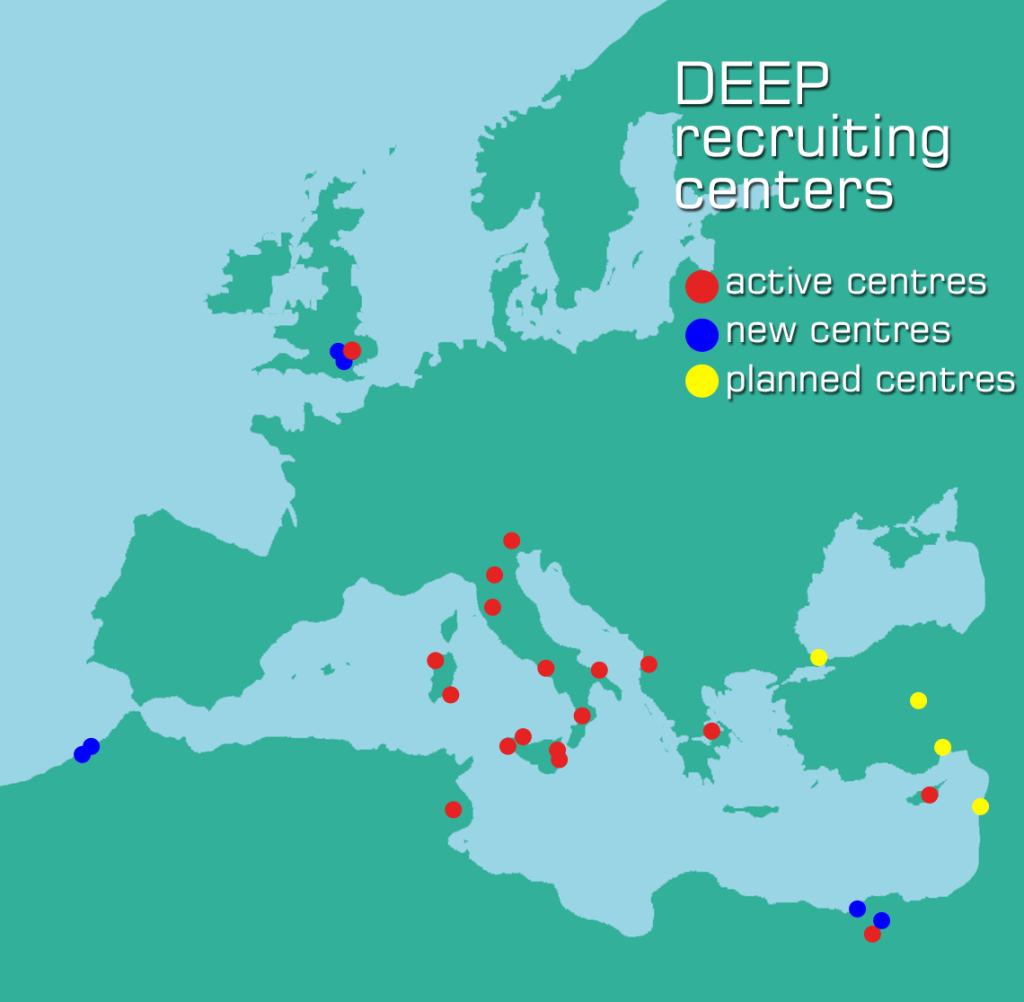
The University Hospital Centre “Mother Teresa” of Tirana (Albania) has received the Site Initiation Visit (SIV) on March 18th, 2015, and is ready to begin the patients’ enrolment.
![]() Cyprus – Waiting for final approval from National BioEthics Committee
Cyprus – Waiting for final approval from National BioEthics Committee
The Department of Medical and Public Health Services of the Ministry of Health of Nicosia (Cyprus) is waiting for the final approval from National BioEthics Committee. The auditing of the documents and the answers, required by the Ethics Committees, were mainly focused on the informed consent and the management of contraception for patients of childbearing age in the study.
![]() Egypt – Cairo is ready to enrol its first patient, authorization process ongoing for Alexandria and Zagazig
Egypt – Cairo is ready to enrol its first patient, authorization process ongoing for Alexandria and Zagazig
The Cairo University Faculty of Medicine has received the site initiation visit (SIV) on March 2nd, 2015 and has already enrolled its first patient into the study, while the Alexandria University Hospital and the Zagazig University Hospital Haematology outpatients Clinic, are both in process of sending the submission package to the Ethics Committee and the Competent Authority.
![]() Greece – Athens is ready to enrol its first patient
Greece – Athens is ready to enrol its first patient
Agia Sofia Children’s Hospital of Athens (Greece) has received the Site Initiation Visit (SIV) on April, 3rd 2015, and is now ready to begin the patients’ enrolment.
![]() Italy – First patients enrolled in Italy
Italy – First patients enrolled in Italy
So far, 8 Italian centres (Bari, Cagliari, Cosenza, Napoli, Padova, Palermo Civico, Palermo Cervello, Sassari) have already started the patients’ enrolment.
![]() Lebanon – Start submission to Ethics Committee and Competent Authority
Lebanon – Start submission to Ethics Committee and Competent Authority
The Nini Hospital centre, located in Tripoli (Lebanon) is in the process of sending the submission package to the Ethics Committee and the Competent Authority. The Centre estimates a potential number of 30 patients to be enrolled.
![]() Morocco – Start submission to Ethics Committee and Competent Authority
Morocco – Start submission to Ethics Committee and Competent Authority
The Hopital 20 Aout, and the Abderrahim Harouchi Children Hospital CHU Ibn Rochd of Casablanca (Morocco) are both in the process of sending the submission package to the Ethics Committee and the Competent Authority.
![]() Turkey – Start submission to Ethics Committee and Competent Authority
Turkey – Start submission to Ethics Committee and Competent Authority
The 3 new centres involved in the study: Hacettepe University Faculty of Medicine Child Health and Disease Hospital (Ankara), the Istanbul University, Cerrahpasa, Medical Faculty/ Paediatric Haematology Hospital (Istanbul) and the Mersin University Faculty of Medicine Child Health and Disease Hospital (Mersin), are all in the process of sending the submission package to the Ethics Committee and the Competent Authority. The three Centres estimate an enrolment of approximately 80 patients in total.
![]() United Kingdom – Waiting for final approval from Competent Authority MHRA
United Kingdom – Waiting for final approval from Competent Authority MHRA
UK has received a favorable opinion from the MHRA, which will become effective when the amendment to the protocol will be presented. This amendment has already been approved by the Coordinating Ethics Committee of the Study, and is ready for the submission to both MHRA and REC. The auditing of the documents and the answers, required by both the Ethics Committees and Competent Authorities of UK, were mainly related to the informed consents and the management of contraception for patients of childbearing age enrolled into the study.